Janssen's Oral Medicine Can Finally Cure Psoriasis
Written by Shaveta Arora, Arushi Sharma
Janssen Pharmaceuticals introduces JNJ-2113, a groundbreaking oral drug targeting IL-23R, offering hope for treating severe psoriasis.
Janssen Pharmaceuticals announced clinical trial results for JNJ-2113, a first-in-class oral IL-23R antagonist drug, treating adult patients with moderate-to-severe plaque psoriasis.
GlobalData predicts $292 million in sales of oral drug targeting IL-23R for psoriasis by 2029.
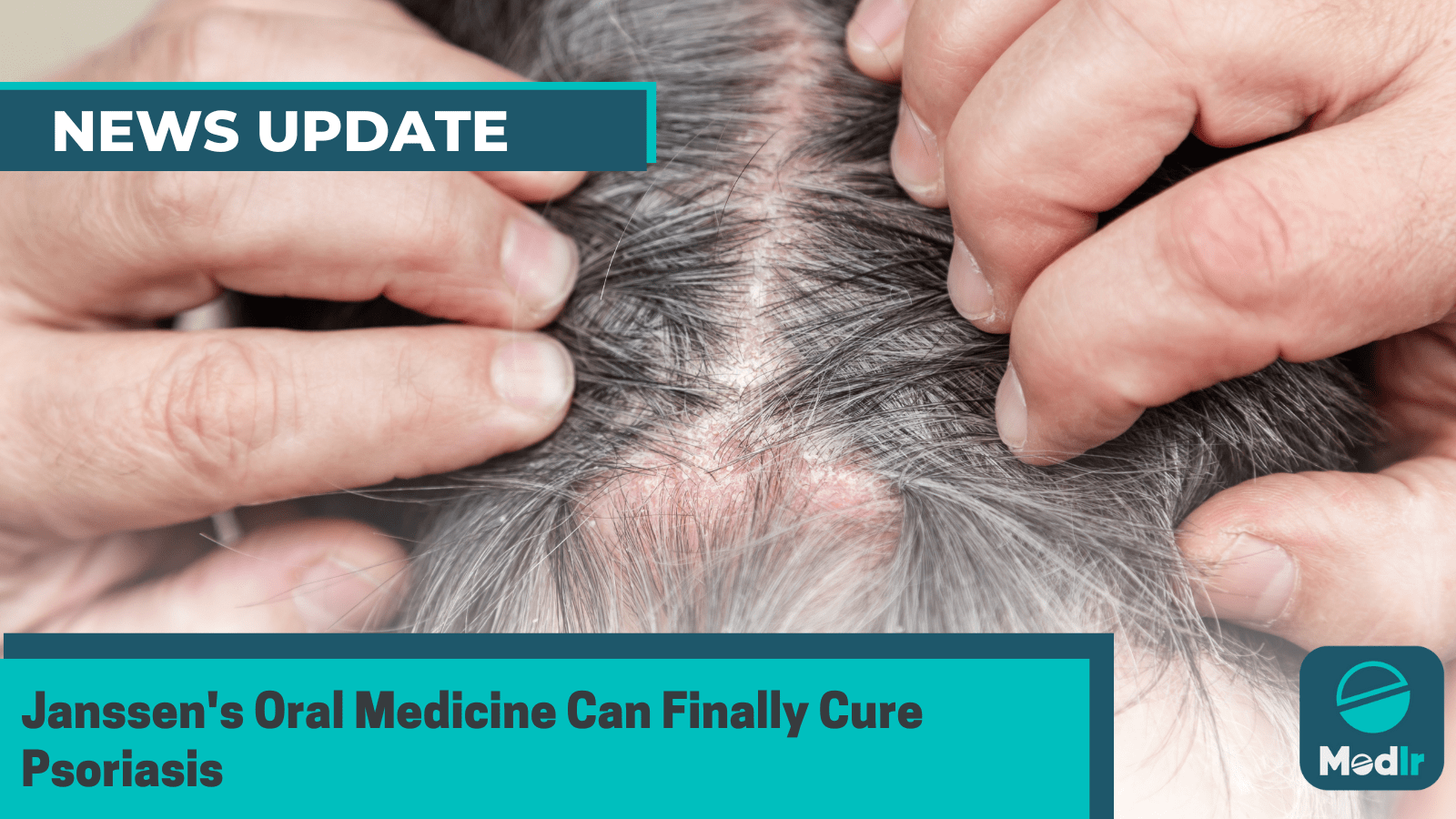
Psoriasis manifests as a persistent skin condition characterized by itchy, scaly patches on the knees, elbows, trunk, and scalp. This condition can lead to pain, disrupt sleep, and impede concentration.
The treatment aims to inhibit skin cell growth, eliminating scales. Options include creams, ointments, phototherapy, and oral or injected medications. Treatment choice depends on psoriasis severity, response to treatment, and self-care measures.
Janssen Creates JNJ-2113, a New Oral Medicine for Moderate to Severe Psoriasis
When compared to the respective placebo groups, phase two clinical trial data demonstrated the potential of JNJ-2113, demonstrating complete clearance and improved skin lesion improvement in participants.
Johnson & Johnson (J&J) further fortified the effectiveness of JNJ-2113 through the outcomes of their randomized, multicenter, double-blind, placebo-controlled FRONTIER 1 (NCT05223868) clinical trial, conducted over 24 weeks in Phase two.
This trial evaluated three once-daily and two twice-daily oral dosages in patients dealing with moderate-to-severe psoriasis, serving as the foundation for the agent's advancement into Phase III development.
At week 16, the Psoriasis Area and Severity Index (PASI) 75 scores were 9.3% for the placebo group, while the JNJ-2113 groups had 37.2%, 58.1%, 51.2%, 65.1%, and 78.6%. PASI 90 scores were 2.3% for the placebo group, while treatment groups had 25.6%, 51.2%, 26.8%, 46.5%, and 59.5% at week 16. These findings indicate dose-dependent responses across primary and secondary endpoints.
As a result, by selectively inhibiting IL-23/IL-23R signaling and the subsequent production of inflammatory cytokines, JNJ-2113 provides a distinct and effective strategy for managing psoriasis.
The encouraging outcomes of this adaptable and user-friendly agent underscore the potential impact of JNJ-2113 on the treatment landscape for moderate-to-severe psoriasis. Nevertheless, caution should be exercised when making indirect comparisons due to variations in clinical trial designs and patient populations.
Treatment groups showed favorable tolerance to JNJ-2113, with no indication of dosage escalation. Common side effects included COVID-19, nasopharyngitis, and upper respiratory tract infections.
JNJ-2113 offers an oral therapy that addresses the IL-23 pathway, potentially enhancing patient outcomes by aligning with patient requirements and choices, unlike existing advanced psoriasis treatments primarily based on injectable biologics.