DeepTek.ai's Chest X-ray AI Gets FDA Clearance
Written by Shaveta Arora, Arushi Sharma
DeepTek.ai, an Indian medical imaging AI company, has secured FDA clearance for its CXR Analyser, an innovative chest X-ray AI solution powered by deep learning algorithms.
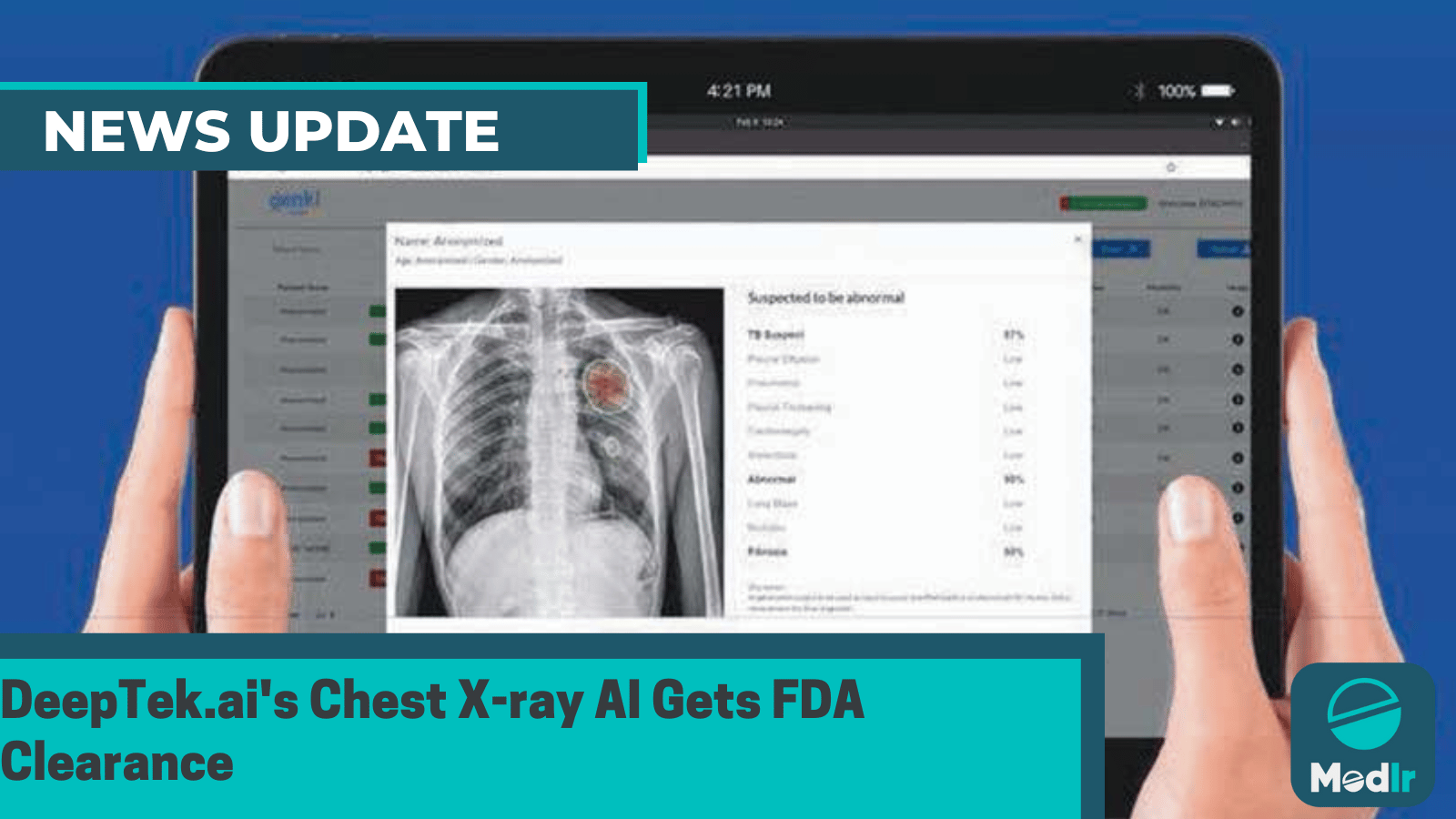
COREek.ai, an Indian medical imaging AI firm, has received FDA approval for its chest X-ray AI solution, CXR Analyser. The technology uses deep learning algorithms to identify, classify, and highlight potential abnormalities in chest X-rays, aiding healthcare professionals in more accurate diagnostic interpretations.
DeepTek's chest X-ray AI analyzes the entire chest region, identifying lung, pleural, and cardiac issues. It's adaptable to various X-ray machine types, including hospitals, mobile units, portable devices, hand-held machines, and ultra-portable units. This AI model detects suspicious lesions in chest X-rays, expediting decision-making and aiding in diagnosing conditions like lung infections, cancer, and chronic lung diseases.
Dr Amit Kharat, Co-Founder, DeepTek.ai quoted, “DeepTek CXR Analyzer v1.0 is a game-changer, reducing radiologist workload by an impressive 30-50 per cent. With FDA clearance, we’re not only accessing the vast US healthcare market but also impacting lives globally. By swiftly identifying lung abnormalities and offering precise mapping, we’re unlocking a world of possibilities for improved outcomes across various medical specialties.”